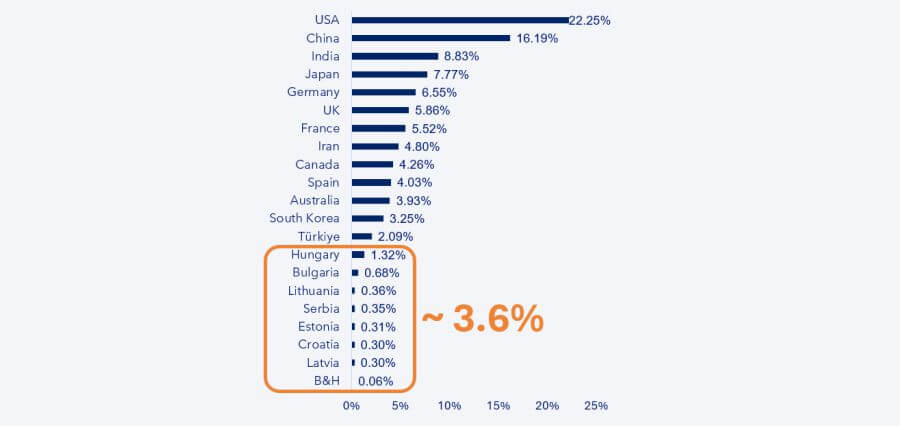
Over the past 25 years, more than 1.2 million clinical trials have been conducted globally, according to the WHO. Until 2020–21, the number of trials initiated each year was steadily increasing. However, despite this growth, the Central and Eastern European region still accounts for only around 3.5% of all trials.
- Hungary, the top performer in our region, ranks only 27th globally.
- Serbia is 50th, Croatia 53rd, and Bosnia & Herzegovina 76th.
- Even Bulgaria and the Baltic countries are in similar positions.
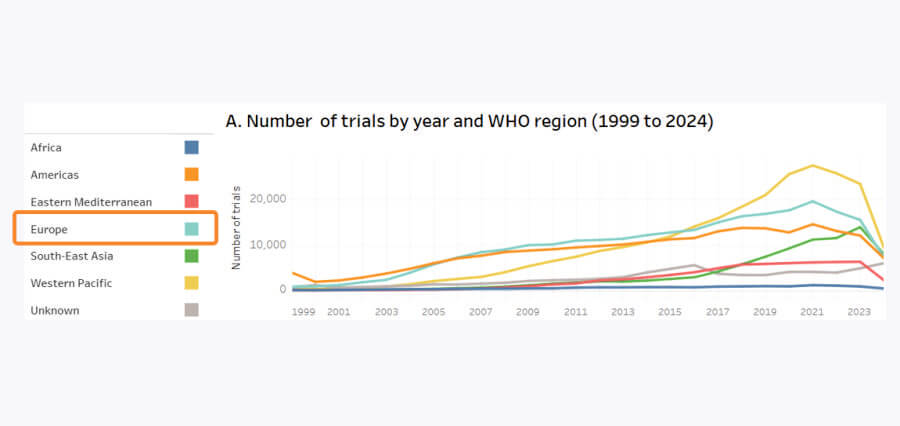
Another trend worth noting is that the global number of trials is now decreasing.
Although trial numbers grew consistently over the past 25 years, in the last 5 years, Europe’s share dropped from 18% to just 9%.
According to the latest IQVIA and EFPIA report, the full impact of the EU Clinical Trials Regulation (CTR) is still unclear. So far, it hasn’t improved Europe’s competitiveness. There are ongoing challenges with the Clinical Trials Information System (CTIS) implementation.

Despite the goal of harmonized standards and simplified regulatory approvals, the reality is that member states show varying levels of readiness and motivation to adopt these changes. The recommendation is clear: the EU and member states should invest in “ready-to-go” clinical trial networks that are open to collaboration with the private sector.
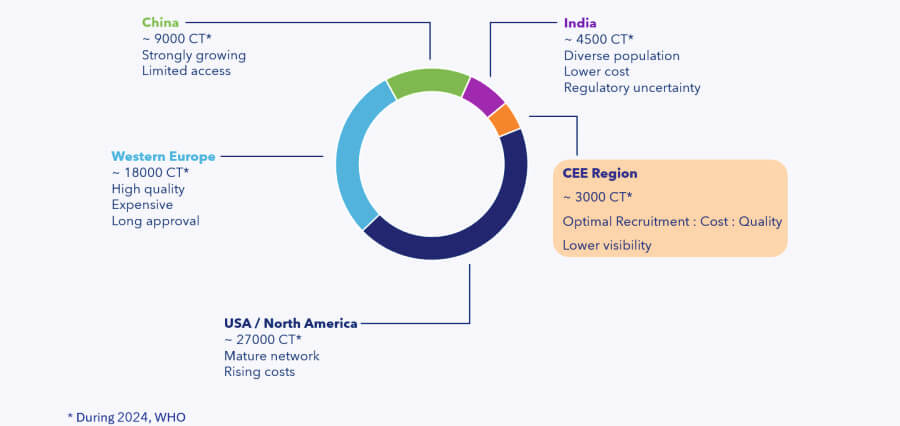
Compared to the leading regions, the CEE region hosts only around 3,000 trials per year (2024 estimate).
Yet it offers one of the best combinations of recruitment speed, cost-efficiency, and data quality.
Each region has its pros and cons:
- The U.S. and Western Europe, while offering high data quality and mature infrastructure, are facing steadily increasing costs, which pose challenges for sponsors.
- China has experienced remarkable growth in clinical trial activity, but access remains tightly controlled, with significant regulatory and operational barriers.
- India offers an enormous patient population and competitive costs, but ongoing regulatory uncertainty continues to impact trial timelines and sponsor confidence.
In contrast, CEE countries are becoming increasingly attractive, offering a well-balanced mix of high-quality data, reasonable costs, and faster recruitment — making them a smart choice for both sponsors and biotechs.
Recognizing these trends, it was decided to launch the Viacrystal Expert Network — a platform built to unlock the untapped clinical trial potential of the CEE region.
The mission of this initiative is:
- To give clients fast access to qualified PIs
- To support early feasibility and influence study design
- And to generate recurring revenue for Viacrystal through consulting services
All the market gaps mentioned earlier can be turned into opportunities through the establishment and active development of an Expert Network.
Given the current low visibility and fragmented information about investigator expertise, we have the chance to create a well-structured, searchable expert directory tailored to client needs and trial requirements.
In the long term, the Expert Network can evolve into a comprehensive service — combining search, consultation, and match — that supports sponsors at every step:
From identifying a potential PI, evaluating their experience, to making an informed, data-driven choice rather than a blind selection.

How are we building this?
Step one: creating a master database.
Over 8 years of feasibility work, Viacrystal has collected a large volume of investigator contacts. After reviewing our trackers, we’ve identified over 1,000 promising leads across about 10 countries and 15 therapeutic areas.
We’ve launched an email campaign to start outreach, explaining the benefits of the Expert Network to these doctors. We’re also searching for professionals on LinkedIn, and we’re relying on support from regional colleagues — to provide insights and recommendations based on your connections.
We’ve already started applying this strategy with colleagues in Bulgaria, and we plan to expand to the Baltics and Türkiye as well.
Right now, we’re still in the early stages, testing different strategies and refining our approach. So far, we’ve onboarded 11 experts, mostly from recent studies, who are already loyal to Viacrystal, and some who joined after seeing the platform from scratch and were excited to be part of it.
At this point, we can offer experts in 7 therapeutic areas across 3 countries. We are also reaching out to previous clients, encouraging them to explore new opportunities in the CEE region.
In our experience operating across the CEE region, we see how therapeutic standards differ not only between countries, but even between cities or hospital networks within a single country. What’s “standard care” in one location might be unavailable or outdated just a few kilometers away.
This complexity often goes unnoticed. It doesn’t show up in feasibility surveys or site feasibility metrics. It only becomes visible through ongoing collaboration with local experts — relationships built over time, not activated ad hoc when a protocol is already locked.
The global clinical research landscape is shifting, and the CEE region stands at a crossroads. While traditional markets face rising costs, regulatory bottlenecks, and recruitment challenges, Central and Eastern Europe offers a rare combination of speed, quality, and cost-efficiency that the industry can no longer afford to overlook.
Viacrystal’s Expert Network is more than a directory — it is an infrastructure investment in regional expertise, built to connect sponsors and biotechs with qualified investigators who understand the nuances of local standards and patient pathways.
By systematically consolidating expertise, fostering early engagement, and creating a transparent bridge between global sponsors and regional investigators, the Expert Network transforms untapped potential into measurable results.
The future of CEE in global clinical research will depend on visibility, readiness, and trust — and the Expert Network is designed to deliver all three.